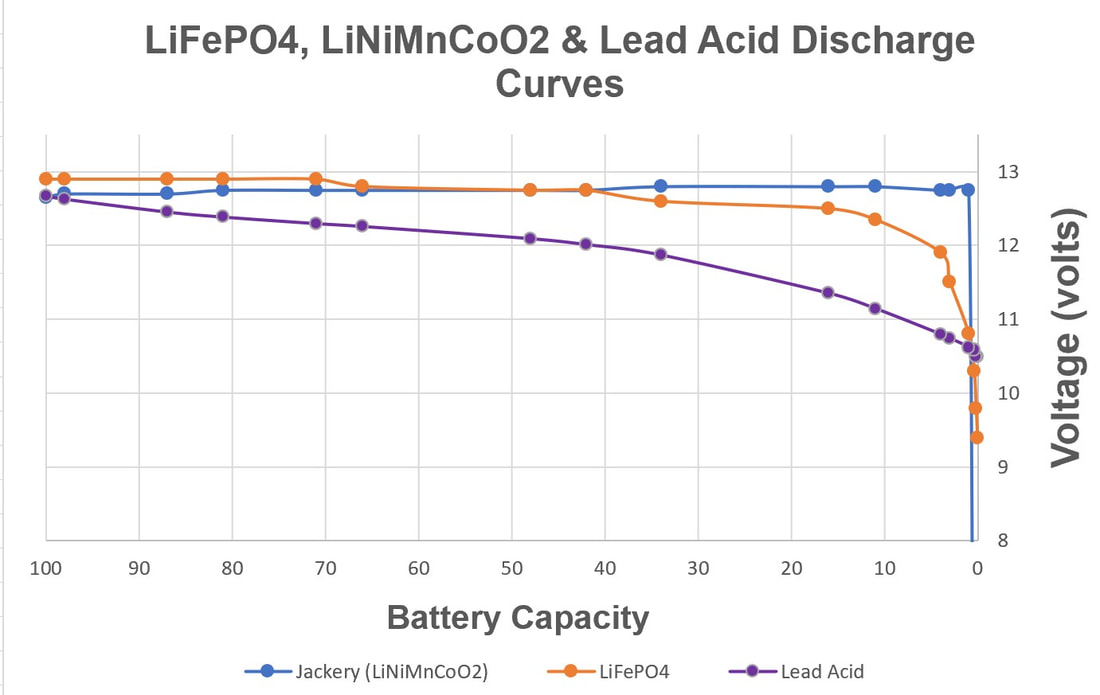
 EBL Voyager 1000Wh Portable Power Station EBL Voyager 1000Wh Portable Power Station Lithium based power sources like Li ion batteries from Li Time, Bioenno Power and Chins along with portable power stations from companies like Jackery, EBL and Bluetti are quickly replacing lead acid batteries when it comes to powering astronomy setups away from AC power. Lithium batteries offer several important advantages over traditional lead acid batteries. First, they have much higher energy densities making them half the weight of a similar capacity lead acid battery. Second, while lead acid batteries should not be discharged more than 50-60% of their rated capacities to avoid damage including loss of capacity and ultimately complete failure, lithium batteries can safely be fully discharged without damage (more on this later). Third, while the voltage of a lead acid battery falls off steeply with depth of discharge, reaching ~12.05V at 50% DOD, lithium batteries have a much flatter voltage vs SOC curve dropping below 12.0V at ~95% DOD. Fourth, lithium batteries have a low self discharge rate of 2-3% per month which means they only need to be recharged once every 3-6 months when in storage. Also, Lithium batteries do not suffer from the memory effect of other battery chemistries so it is not necessary to fully recharge them after use. Finally, lithium batteries will supply more of their rated capacity in cold temperatures compared to lead acid batteries with some estimates indicating as much as 2X the capacity compared to lead acid below 32F. However, with this new source of energy comes a lot of confusion and downright wrong information on the astronomy forums. To correct this misinformation I decided it was time to provide a review of the basics of lithium battery technology and to correct the most common misconceptions that I see posted on these forums. Over the last three years I have immersed myself in lithium technology by both doing a literature search on the subject and by talking directly with multiple suppliers of lithium batteries and power stations to gain a fuller understanding of the technology and manufacturing methods. I visited the Battleborn factory technical team and toured the factory floor to see and hear how they design, test and assemble their batteries. Most importantly, working with multiple suppliers I have conducted my own testing of LiFePO4 batteries from 6 different vendors to collect data for myself to compare to manufacturer's specifications and to test out some common misconceptions. This Blog is a summary of the entirety of my efforts in this regard. 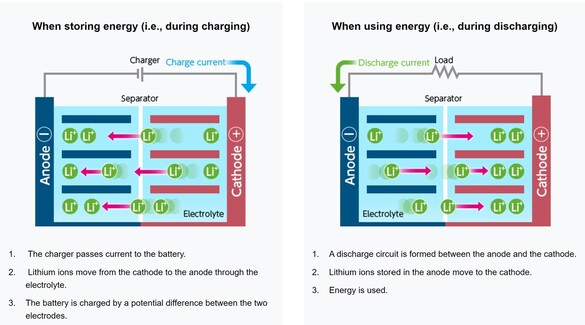 Li ion battery charge and discharge cycles from Toshiba Li ion battery charge and discharge cycles from Toshiba Lithium Battery Basics There are many different lithium battery chemistries that have been developed, each with their own specific advantages and disadvantages. These include LiCoO2, LiMn2O4, LiNiMnCoO2 (NMC), LiFePO4 (LFP), LiNixCoyAlzO3 (NCA) along with other less common chemistries. One of the first misconceptions is that Li ion is a specific type of lithium chemistry when in fact it is simply the general name given to all of the above Li chemistries. Li ion batteries are so called because they use Li ions to generate electric current. Like lead acid batteries, Li ion batteries consist of multiple cells in series to achieve the desired voltage and capacity of the battery. Li ion cells consist of a graphite anode, a lithium metal oxide cathode (such as the ones listed above), a separator, an electrolyte and a casing. During discharge the Li ions flow from the anode to the cathode releasing electrons which supply power to the load. During the charging cycle, Li ions flow across the separator to interstitial positions between the planes of carbon in the graphite anode via a process called intercalation. The metal oxide cathode material is the main difference from one Li battery chemistry to another. Unlike lead acid batteries, Li ion batteries have an internal electronic component called a Battery Management System (BMS) that is designed to make sure that the battery operates safely and is protected from unsafe or potential damaging situations. The BMS has multiple functions. It protects the cells from over voltage, over charging, over discharging, over current, and short circuits. It also balances the internal cell voltages to keep the cells in balance so that they all are charged to the same voltage. Cell balancing happens at the high end of the charge cycle which is why you will find it takes much longer to charge a Li ion battery the last few percentage of the state of charge (SOC) than it does through the bulk of the charging capacity. It is also why it is important to use a Li charger which will charge the battery to the correct voltage which is higher than the typical charging voltage with a lead acid charger. Also, on many batteries but not all, there are one or more internal temperature sensors which are connected to the BMS to stop it from charging or discharging when outside the manufacturer's specified temperature operation limits. If the battery does not have this feature it is up to the individual to make sure that the battery is not charged below 32F. 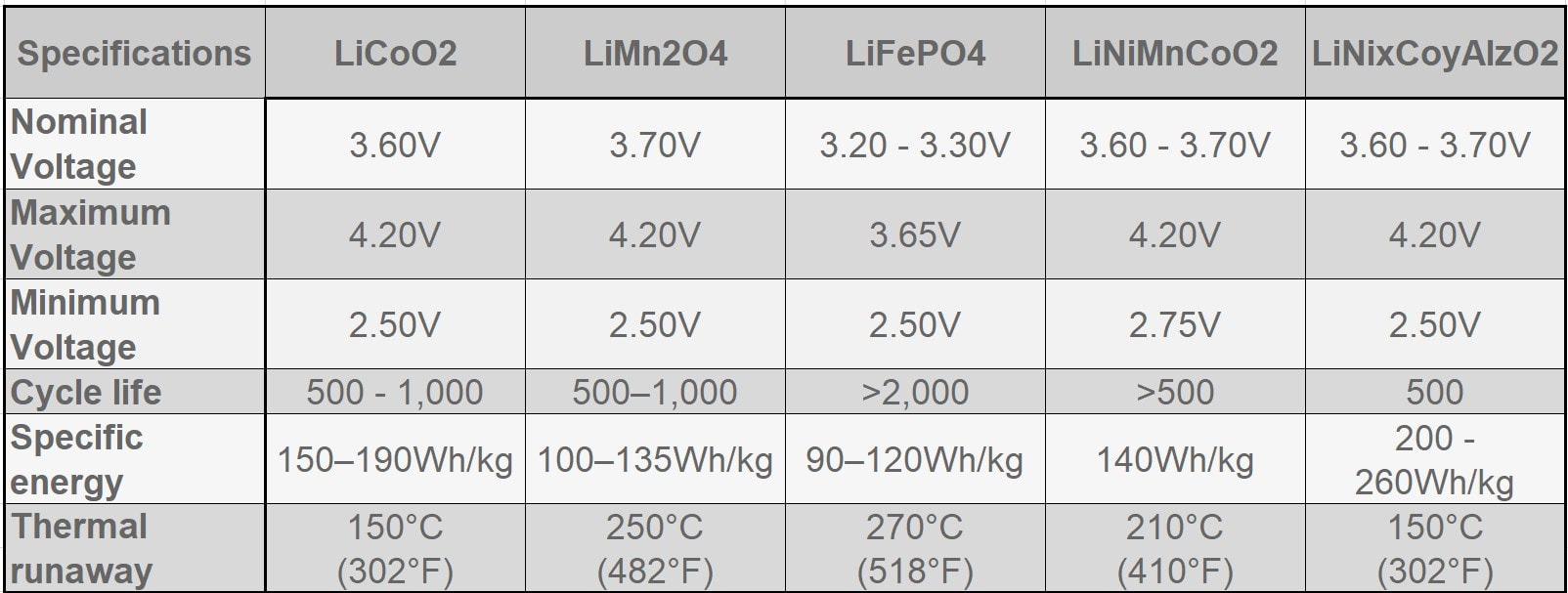 Key lithium chemistry properties taken from Battery University & Other Sites Key lithium chemistry properties taken from Battery University & Other Sites The two most common Li ion chemistries found in the batteries and power stations of interest to us are LiFePO4 and LiNiMnCoO2 which we will focus on in this Blog. Looking at the table below, one can see that LiFePO4 has a nominal voltage of 3.2V which means that 4 cells in series produce a battery with a voltage of 12.8V, ideal for a "12V battery." A 100Ah LiFePO4 battery will typically have 4 3.2V cells with 100Ah capacity each in series to achieve both the specified voltage and Ah capacity. LiFePO4 also has the highest thermal runaway temperature among the most common lithium chemistries. It also has the highest discharge cycle life with 2K to 5K full discharge cycles. This is why you will find that LiFePO4 is used in lithium batteries designed to replace 12V lead acid batteries. LiNiMnCoO2 has an energy density as much as ~2X that of LiFePO4 cells. This is why LiNiMnCoO2 is commonly found in applications where weight is critical including ebikes, power tools, electric cars and portable power stations. While its thermal runaway temperature is lower than LiFePO4, it is still quite high. With a nominal cell voltage of 3.6V there is no way to combine cells to achieve a voltage consistent with a 12V battery. For instance, 3 cells in series produce 10.8V which is too low while 4 in series produce 14.4V which is too high for 12V systems. This is why portable power stations with LiNIMnCoO2 cells have voltage regulation. If you were to look inside one you might find 4, 6 or more cells in series which would produce voltages of 14.4V, 21.6V, or higher which are then regulated to provide an output voltage of ~12.8V consistent with other 12V power supplies. Typical voltage versus depth of discharge data show the key differences among the different power sources. Lead acid batteries have a relatively steep discharge curve with the output voltage falling below 12V at 40% capacity. LiFePO4 on the other hand has a slow voltage drop remaining above 12.0V until 95% of the capacity has been used. Since LiNiMnCoO2 requires voltage regulation as found in power stations like the Jackery and other brands, the voltage remains flat all the way until the internal battery BMS shuts off the output with 0% capacity remaining. Lithium Batteries vs Power Stations When it comes to Li ion power there are two very different options. The first is a stand alone Li battery which is designed and packaged to replace a lead acid battery. As such, it looks identical to a lead acid battery with the lithium cells enclosed in an ABS case similar in size to an equivalent capacity lead acid battery. It has a positive and negative terminal just like a lead acid battery. As described above, the Li battery also has a BMS to provide for safe operation and long life of the battery. Stand alone batteries use LiFePO4 and come in many different capacities from a few Ahs to hundreds of Ahs. The second Li power option is a Li ion power station. A power station not only provides 12V dc power, but also has an internal pure sine wave inverter to provide AC power via one or more outlets. It also has multiple powered USB ports to charge phones, tablets and even laptops. All portable power stations that I have seen come with a cigarette output port with a maximum current of 10A. Some come with additional dc power ports with a variety of connector types (5.5 x 2.1mm, 5.5 x 2.5mm, 6.5 x 4.1mm, etc.) usually rated 3A - 7AA. Power stations come with an AC charger to recharge the device from a wall outlet. They also have an internal solar charge controller which accepts input from one or more solar panels (not typically included) to recharge the power station via the sun. Power stations include an internal meter and display to show the state of charge (SOC) and power during discharge and recharge operations. Power stations also have an internal BMS which performs the same functions as in the LiFePO4 battery. Like the stand alone LiFePO4 battery discussed above, power stations come in a variety of capacities typically stated in Wh from ~200 to over 2k Wh. Most power stations use LiNiMnCoO2 cells due to their higher energy density which helps keep the weight down since power stations are meant to be portable devices. There are however, several power stations which use LiFePO4 cells. With LINiMnCoO2, the dc output is regulated since, as discussed above, the cell voltages do not add up to 12.8V like they do with LiFePO4. Lithium Ion Cycle Life and Calendar Life One of the biggest myths of Li ion batteries is the one which says you cannot discharge a Li ion battery more than 80% or 90% without damaging it. This is patently false. Li ion batteries and power stations are designed to be fully discharged. That means you have access to 100% of the rated capacity without risk of damaging the individual cells inside and without degrading the rated number of discharge cycles of the battery or power station. For instance, if you check the web sites for Battleborn, Ampere Time (re-branded as Li Time), Chins or Lithionics you will see that they all specify their cycle life in terms of 100% depth of discharge (DOD). Battleborn specifies 3-5K cycles, Ampere Time 4K + cycles, Chins 2K+, and Lithionics 2K cycles. And, after that the battery will still retain 75 - 80% of its original capacity. In the power station category Jackery with LiNiMnCoO2 cells specifies >500 full discharge cycles after which one can expect 80% of the original capacity. In contrast, as most people know, a lead acid battery should not be discharged below 40% of its capacity otherwise sulfates will form on the plates reducing capacity and eventually shorting out the cells. In contrast, the BMS inside Li ion batteries monitors the individual cell voltages inside to make sure that no cell's voltage drops below the minimum value shown in the table above, typically 2.5V. When any cell reaches the minimum the BMS shuts the battery down to prevent cell damage. So the battery is designed to provide its full rated capacity without any cell's voltage dropping below this safe minimum voltage. Now it is true that you can extend the cycle life by not fully discharging a Li ion battery. For instance, discharging a Li ion battery to only 50% DOD can improve the cycle life by almost a factor of 3. But then you would need to purchase and handle two batteries instead of one to provide the power you need for your equipment. A good compromise might be to size the battery you purchase such that you only need to discharge it by 80% - 90% just to be on the conservative side. Calendar life of a battery refers to how long it will last if stored under ideal conditions of state of charge, temperature and humidity. This is independent from cycle life. Typical calendar lifetimes are quoted as 10 years, although Li ion batteries have not been around long enough for a significant amount of data to be collected to confirm this estimate. By contrast, the calendar life of a lead acid battery is usually quoted as 4 -6 years. The main mechanism for calendar aging is the growth of passivation layers at the interfaces between the electrolyte and the cathode and anode. This growth depletes the amount of available cyclable lithium. Both high temperatures and high SOC can accelerate calendar life degradation.  Battleborn 100Ah LiFePO4 Battery Battleborn 100Ah LiFePO4 Battery Operating Temperatures All battery types have specific temperature ranges of operation and Li is no different. Typically, lithium batteries can be discharged safely down to -4F (-20C) and up to 120F (49C) to 140F (60C) depending upon the manufacturer's spec. Charging of lithium ion batteries is only permissible at temperatures above 32F (0C) unless the battery has an internal heater and temperature sensor which will allow charging below an ambient temperature of 32F but not below an internal temperature of 32F. Charging a Li ion battery bellow 32F will cause damage to the internal cells by causing some of the Li ions to plate as Li metal on the anode surface rather than intercalating between the carbon planes in the graphite anode. This happens because there is not enough thermal energy to drive the Li ions into the graphite structure. This reduces the capacity of the battery and if done too often can lead to the formation of Li metal dendrites which short across the separator to the cathode causing catastrophic failure of the battery. Li ion batteries also have a maximum temperature at which they can be charged which ranges from 113F (45C) to 131F (55C) depending upon the manufacturer's spec. The battery itself produces heat during discharge which combined with a high ambient temperature can cause swelling of the cell and venting. A battery with an internal temperature sensor connected to the BMS will prevent charging and discharging out of the battery's specified temperature range. It is also possible to damage a Li ion battery by trying to charge it too fast with too high of a current. Manufacturer's spec the charging current between 0.2 and 0.5C meaning, 20A or 50A for a 100Ah battery (10 to 25A for a 50Ah battery, etc.). Once again the reason has to do with the mobility of the Li ions. If they arrive at the anode too quickly, i.e. at too high a charging current, they can begin to pile up at the surface of the anode instead of diffusing into the graphite lattice. Check the manufacturer's specification for temperatures of operation and charging and discharging maximum currents. Another common misconception about Li ion batteries is that they perform poorly at cold temperatures. The fact is that all battery chemistries including lead acid have less capacity at temperatures below freezing. This is simply the result of the fact that batteries work by the process of diffusion from one electrode to the other. Diffusion rates are a function of temperature with rates decreasing significantly at low temperatures. However, as indicated above, Li ion batteries retain more of their capacity at low temperatures than lead acid batteries, hence they actually perform better at low temperatures with the caveat that they cannot be charged below freezing. Charging & Storing a Li Battery Many people make the mistake of trying to use a lead acid battery charger to re-charge a Li battery. The problem with this is that a lead acid battery charger will stop charging a Li battery before it reaches the required 14.4 to 14.8V required to fully charge the cells inside. This results in charging the battery to only 70 - 80% of its capacity. Not only that, but the typical BMS will not perform the important function of cell balancing until the top end voltage is reached. Over time, this will degrade the overall capacity of the battery even if you later use a charger capable of charging to 14.4V. Another source of confusion when charging a Li battery can result when the battery is fully discharged (SOC at 0%) to the point that the BMS shuts down the output. If you put a volt meter on the battery after the BMS has shut it down you will measure a voltage of 0V. Many battery chargers, including some with a Li battery setting, will not charge the battery if they see 0V. Make sure that the charger is one which specifies 0V charging like this one from Li Time or similar. A trick to get a non 0V charger to work (still needs to have a Li setting) is to connect any 12V battery or jump starter to the Li battery momentarily to "wake up" the BMS. It only takes a momentary contact making sure to connect the positives to each other and the negatives to each other just like jump starting a car battery. It is always important to keep a Li ion battery from getting too hot and for we astronomers that means keeping out of direct sunlight on the viewing field. This is especially true when charging the battery as internal heat is produced when charging (also when discharging) a Li battery due to its internal resistance. The added thermal burden of high ambient temperatures and direct sunlight can raise the internal battery temperature and result in cell damage. I always tuck my battery or power station in the shield behind my solar panels to keep it cooler. If a Li battery needs to be stored for an extended period most manufacturer's suggest storing it at a 50% SOC although at least one company, Battleborn, says to store it at a 100% SOC. Based upon the fact that published literature show that a high SOC is more stressful to a Li battery it is probably best to follow the lower SOC suggestion of 50%. Because Li batteries have a low self-discharge rate of 2-3% per month they only need to be recharged every 3 - 6 months. To make sure that the BMS balances the internal cells you would need to recharge it to 100% SOC and then discharge it to the whatever SOC you plan to store it at, say 50%. Li Battery Safety While lead acid batteries can cause fires and explode if not properly handled, lithium batteries have received a lot more media attention for dramatic failures in recent years. The term "venting with flame" was coined to describe thermal runaway events where the can containing the cell components breaks and fire escapes. Despite all the media attention, lithium batteries are quite safe when handled and operated properly. The Occupational Health and Safety Administration points out that lithium batteries are "generally safe and unlikely to fail, but only so long are there are no defects and the batteries are operated safely." Lithium batteries fail for one of several reasons. Manufacturing defects like the presence of tiny metal particles in Sony batteries in the 90s can cause an internal short and resulting failure. Manufacturing processes have improved greatly since then, but it is probably a good idea to purchase a lithium battery from a supplier who has been around for a while and has a good reputation as one would expect them to source high quality components and use proper assembly and testing procedures. Like lead acid batteries, lithium batteries can fail when damaged from falls, punctures or excessive vibrations. They can also fail from improper use such as charging below the low temperature cut-off, charging with too high of a current, a short circuit or excessive temperatures. The internal BMS is a major safety feature to help insure safe operation. But it can only prevent operation outside the specified temperature limits if it has one or more internal temperature sensors which not all lithium batteries have. In summary, if handled and operated properly a quality lithium battery should not be any more dangerous than a lead acid battery. Many of the Li ion battery failures are from devices like cells phones, tablets and laptops which use LiCoO2 as the cathode material because it has one of the highest energy densities. However, it also has the lowest thermal runaway temperature as well. One should not draw generalized conclusions about the safety of LiFePO4 and LiNiMnCoO2 batteries and power stations from failures in devices using LiCoO2. Lithium vs Lead Acid Battery Cost Comparison Another popular misconception is that Li ion batteries cost more, even much more, than lead acid batteries. The fact is the total cost of a lithium battery is much less the cost of an AGM battery. A check on Amazon shows 100Ah AGM deep cycle batteries selling for $165 to $204 while name brand LiFePO4 batteries from Chins and Li Time sell for $320 and $350, respectively. Knowing that lead acid batteries should not be discharged more than 60% while LiFePO4 batteries can be safely discharged 100% one would need 1.7 100Ah AGM batteries to supply the equivalent energy as a single LiFePO4 battery. So the actual up front cost comparison is $3.20 to $3.50 per Ah for LiFePO4 versus $2.75 to $3.40per Ah for AGM. Now we must take into account the useable life of each battery type. For LiFePO4 the most conservative estimate is 2,000 full discharge cycles while the most optimistic estimate for an AGM is 750 cycles. Taking this into account the total cost per Ah over the full cycle life is less than 0.2 cents for LiFePO4 and 0.4 cents for an AGM battery. So a LiFePO4 battery costs less than half that of an AGM battery over the full expected cycle life of the battery. Another way to look at this is the calendar life of the battery. As we mentioned above, the calendar life of a LiFePO4 battery is 10 years while that of an AGM battery is ~5 years, nearly half as long. So, no matter how you compare the two, a LiFePO4 battery is a better value than a lead acid AGM battery these days. Now the cost comparison is different when we talk about Li power stations like those from Jackery and EBL. The EBL 1000Wh power station sells for $490, is expected to provide 500 full discharge cycles and provides 83Ah per cycle. The total cost comes to 1.2 cents per Ah. Power stations are more expensive options compared to stand alone LiFePO4 batteries because they provide many more features besides dc power including pure sine wave inverted AC power, multiple USB power ports, an internal solar charge controller, internal meter and display, etc. Summary and Conclusions As we have seen, Li ion batteries have many advantages over lead acid batteries which is why they are fast becoming the batteries of choice for amateur astronomers. A 100Ah LiFePO4 battery with a capacity of 1280Wh can be expected to provide power to an astrophotography or Electronically Assisted Astronomy setup requiring 64W of power (5Ah) for a total of ~20hrs before needing to be recharged. They are in fact cheaper than lead acid and perform better than lead acid at all temperatures. When handled and operated correctly Li ion batteries are safe. To achieve the maximum useable life and capacity, as well as, prevent damage to the battery or even a catastrophic failure, always follow the manufacturer's recommended specifications and operating instructions. Links are affiliate links which help to support this web site at no cost to you. If you purchase a lithium battery or portable power station as a result of reading this Blog I ask that you please use my links for your purchase.
Get 3% Off any LiFePO4 battery from Li Time using coupon code CURTISM and the Li Time link below LiFePO4 Batteries Li Time (formerly Ampere Time) Amazon amzn.to/3M9sbv8 Li Time 's Web site www.amperetime.com/?ref=VlYrkkdj Chins amzn.to/3nEZBYc Dakota Lithium amzn.to/3nDhp6f Battleborn amzn.to/3Zreyug Bioenno Power amzn.to/3K3SAIb Portable Power Stations Jackery Amazon amzn.to/3G5Xwec Jackery's Web Site www.jackery.com?aff=196 EBL amzn.to/3nyNc8i Bluetti amzn.to/40vemvb
0 Comments
Leave a Reply. |
Categories
All
Archives
January 2024
|



 RSS Feed
RSS Feed